- How will you know what substances you have put in it?
- How do you connect the store’s barcode system to your sample data?
- How do you track your samples outside of the store?
On its own, an automated sample store solves the problems of sample integrity and rapid retrieval. But it doesn’t provide solutions for easily searching through your samples, managing the sample life cycle or laboratory sample tracking outside the store.
An automated store will:
- Store and retrieve samples at much higher throughputs and without human error
- Know exactly where to place samples so you don’t have to search freezers for space
- Improve the integrity of your samples by keeping them in secure, undisturbed environment under monitored conditions
- Track sample barcodes in and out of store
- Run unattended, freeing up scientists to get on with more important tasks
However, in order to know what has happened to samples while they are not in the store; or to let you search what’s in there by using sample criteria, you need another system that talks to your automated store’s database.
You can use a spreadsheet or sample management software, but before deciding there are three important points to consider:
1. Data integrity and compliance
Having purchased an automated sample storage to ensure sample integrity, it would be inconsistent not to match this with an equal concern for the integrity of your sample data.
The US Food and Drug Administration (FDA) requires an “audit trail” for data, meaning that data must be recorded when an entry is created, modified, or deleted. The data must also retain its integrity, without being corrupted, and must be secured to prevent tampering.1
Meeting FDA requirements with an in-house spreadsheet presents problems of accuracy: a spreadsheet requires constant manual updates which are a recognised source of errors. Hosting the spreadsheet so it is generally accessible but it provides the required data security is a challenge. An in-house spreadsheet also requires a custom interface to each item of lab equipment (such as an automated sample store) and a lot of testing to ensure the spreadsheet and interface are functioning correctly.
Even when companies do not handle biospecimens, legal agreements on samples shared with other organisations are common. It can become a complex problem to track, log and authenticate access to samples, expiry dates and agreed disposal.
Most sample management software or LIMS systems are designed to meet or exceed FDA requirements and offer web interfaces for easy data access while maintaining database integrity. Software like Titian Mosaic is set up to handle authentications, automatically log actions, restrict access to certain samples and manage expiry dates in line with best practice.
2. Search tools
One of the functions of sample management software is to connect a store’s database with sample data. This allows you to search by sample criteria, which – unlike a barcode – is information used by scientists. For example, in order to identify materials with a particular concentration and automatically draw up a list of which sample barcodes to request from the store.
Titian's Mosaic goes beyond this to provide access to information about the entire sample life cycle. This includes intuitive searching capabilities that make planning your experiments or removing expired samples very easy.
Read our blog “The benefits of robust inventory search” for more details.
3. Tracking samples outside your store
In life sciences, you don’t just need to know which samples you have in a store, but what happens to each sample once it leaves the store to be prepared for an assay or moved to another location:
- Has it been diluted?
- Does it need replacing?
- Does it require special handling?
- Has the temperature changed?
- Do you know where it is now?
Lab processes often have a lot involved, and it is difficult to track all sample movement and locations as they happen across storage, liquid handling and lab instrument workflows.
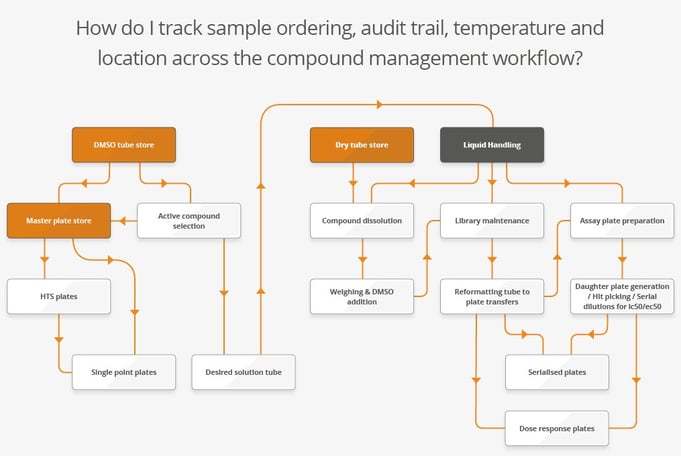
Processes outside the store often involve further automation, such as a liquid handler for efficient and accurate dilutions. These instruments record the actions they perform – but are you able to connect this with the rest of your sample data? For instance, to know that the sample volume has been depleted. If your process changes, this will mean additional programming and testing to be sure a spreadsheet can reflect this.
Mosaic SampleBank software allows existing and new lab equipment to be easily integrated into the data flow. In fact, it makes managing automation very much easier as it can all be done within the one interface, without the need to write or maintain custom scripts. Instead you create a Mosaic order for samples from your new store, and you can add actions to this order, as required. These actions are automatically tracked and logged around the sample life cycle. Changes in process can also be managed from the same interface.
Managing automation and workflows within Mosaic is also very much more efficient: multiple assays can be consolidated into one order for efficient processing; instrument use is optimised and errors from manually transferring data files between systems are eliminated.
The benefits of integrating your automated store
Connecting automated sample storage with a good sample management system quickly gives a return on investment (ROI) that includes:
- Ordering is massively speeded up
- Reducing the time taken to find samples
- Providing an unbroken chain of custody (audit trail)
- Inventory searching is easy and up to date, avoiding duplication of samples
- Sample management software like Mosaic allows you to consolidate many assay runs into one, with attendant reagent and time savings
- Incorporating automation management in workflows provides efficient scheduling, adding to time and cost savings
- Such integrated workflows also save time and reduce errors when transferring information between systems, and provide results faster
- Turnaround time for assays is speeded up, and at the same time data quality is improved
REFERENCE
1 US Food and Drug Administration (FDA). Data Integrity and Compliance with Drug CGMP Questions and Answers Guidance for Industry. Silver Spring, MD; 2018.
Want to know more?
Download our free white paper: The Essential Guide to Managing Laboratory Samples
Watch a tutorial from SLAS 2018 where Titian, HighRes Biosolutions and Genedata present an integrated end-to-end screening solution where scientists can:
- design and order assay plates in Titian’s Mosaic sample management software
- accept and run the fully automated screening process on a HighRes system
- trigger the automated data analysis and approval in Genedata Screener
In any case, consider talking to one of our Titian experts – there is a better way to manage your data flow!
