A three-part guide to exploring common problems experienced in the management of samples
This Essential Guide to Managing Laboratory Samples is a three-part piece exploring common problems experienced in the management of samples and how, for any scale of operation, significant efficiency and accuracy benefits can be gained using dedicated sample management software.
The authors have drawn on Titian Software’s extensive experience of implementing sample management systems for organisations of all sizes. The principles of good sample management hold for any life sciences laboratory, from a small academic laboratory with a single fridge and freezer to large multiuser enterprise environments with multiple stores to manage.
Although freezers may be your primary storage unit, they are not necessarily the only type. Consider the following:
It is a challenge to track such diversity and capture each level of the hierarchy. Some tools aimed at managing your freezers do just that: help you to maintain a list of freezers and their contents. But if your storage is more complicated than that , your tracking system needs to accurately represent the hierarchy of your laboratories, storage areas, shelves, and so on, from top to bottom.
Monitoring free space in your storage areas by visual inspection can be a chore. Knowing how much free space you have and in which stores is difficult to achieve with spreadsheets, but sample management software should make it quick to run a report on your store use. Having easy access to this information allows you to plan for new storage as needs arise, or to perform housekeeping of your inventory.
Example of software that displays all of your storage locations
Even when you know there is available space in a freezer, you might not know exactly where the space is. It is a frustrating exercise pulling racks from a LN2 dewar trying to find room for a few tubes you have just made.
A good freezer management system will allocate free spaces to every new item, taking into account the type of container you are trying to store. This means lab scientists can just follow the list and place items. Of course, your software should also give you the option of assigning positions manually as well.

Monitoring free space in your storage areas by visual inspection can be a chore. Knowing how much free space you have and in which stores is difficult to achieve with spreadsheets, but sample management software should make it quick to run a report on your store use. Having easy access to this information allows you to plan for new storage as needs arise, or to perform housekeeping of your inventory.
Wasting space on redundant materials
One of the biggest consumers of storage space is maintaining redundant stocks from old projects, or items belonging to scientists who have since moved on. Poorly labelled and described inventory leaves you in the position of not knowing what is valuable and what can be disposed of. It costs to maintain these stocks which may have no benefit. A sample management system tracks users and expiry dates, giving you the opportunity to rationalise your materials and make housekeeping simpler in the future.
Fragmentation
Sample storage within an organisation often becomes fragmented over time, with samples – and storage space – spread over a large number of freezers, when these samples could be managed more efficiently in fewer stores. When moving samples to defragment the stores and consolidate boxes, the challenge is to accurately record the samples’ updated positions, without introducing errors. It is difficult and very time consuming to do this manually. Good sample management software should provide tools incorporating barcode readers to simplify the process and avoid errors. The best software helps to prevent fragmentation from happening by suggesting open spaces for placing of new tubes as they become available, keeping track of samples right down to their X-Y location in a box.
![]() Being certain you have the right sample isn’t easy when you have to wipe the frost off to try to read the handwriting on the side of a tube. Barcoding is much more accurate and error-proof for labelling, but you need a system that will help you to use barcodes.
Being certain you have the right sample isn’t easy when you have to wipe the frost off to try to read the handwriting on the side of a tube. Barcoding is much more accurate and error-proof for labelling, but you need a system that will help you to use barcodes.
The benefits of barcoding all of your labware are significant, including:
In our experience, many laboratories that do not use barcoding would like to do so but are unsure how to start. Common questions are:
Sample management software should give you tools to easily manage barcoded labware, as well as your existing unbarcoded stock. It should:
A site closure, where hundreds of freezers contained untracked, unidentifiable research material, drove one company to evaluate sample management software. Without an electronic tracking system, this unidentifiable material could not be transferred to other research groups who could use it. Their system, based on Titian’s Mosaic FreezerManagement, now has:
Knowing the locations of your tubes and plates is only useful if you also know what they contain, so the recording of materials and their attributes is essential to good sample management.
However, despite the best efforts of an organisation to register materials and record their attributes, if materials are described in inconsistent ways the value is limited. It becomes almost impossible to perform a search for a sample without knowing every possible synonym. Figure 1 shows a real example of the diversity of descriptions captured for a single reagent type:

Image: How many ways can we say "Genetecin"?
This is a common problem with databases and spreadsheets. What is needed is software that will allow you to define substance attributes easily, and enforce accurate and consistent description using controlled vocabularies. As well as drop-down lists of values, other property types should be available - such as dates, numeric fields, and free text where appropriate. An administrative user should be able to set all of this up via an easy graphical user interface.
Recording the amount of a sample will use different units depending on the material type – for example mg for solid materials, “number of slices” for a tissue block, mL or µL for solutions. However, the vocabulary of amount units needs to be controlled to ensure that amounts are recorded in a standard way.
A typical lab problem is forgetting to update the remaining amount after a quantity has been removed from a tube. Sample management software should automatically decrement amounts for you when you create a new tube from an existing one, with the transfer out being recorded in the audit trail.
If the amount of a sample isn’t recorded and tracked, you may not know if you have sufficient for your needs without manually inspecting your inventory, which could be time consuming. Worse than that, you may retrieve a tube at the point of use and find it is empty. Wouldn’t it have been better to know in advance so that stocks can be replenished?
When using biological substances, the concentration units being recorded will depend on the material type – for example mg/mL, cells/mL, mM, OD. But even for a single type of material, scientists may express concentration in a variety of different ways. Unless you have some means of providing a controlled vocabulary of concentration units, you may end with information that is difficult to interpret. Figure 2 shows a real example of the proliferation of ways of recording concentration where standard units were not enforced.

Image: Different ways of quantifying the concentration of cells when no control is in place
Even if a concentration is captured correctly, when a sample is diluted the record needs to be updated accordingly. In practice, this is not always done if it is not simple to do. To make lab tasks easier, good sample management software should understand concentration units and the relationship between them. For example, when a lyophilised protein or DNA sample is resuspended, the concentration should be automatically set, or when a sample is diluted by adding some more buffer, the resulting concentration change should be automatically calculated and recorded.
Many materials, such as siRNA and some purified proteins, degrade with repeated freeze-thaw cycles. Tracking these freeze-thaws is a headache for many scientists.
Software can take care of this tracking for you. Ideally, it should allow you to specify a temperature for each of your stores and locations, and automatically record a freeze or thaw event as your materials are moved into, or out of, a freezer so you no longer need to track this separately.
Having a well-described inventory allows more efficient use of the valuable materials that may be held in your organisation. To achieve this, your inventory needs substance types and properties to be defined and rationalised, together with easy to use query tools. However, being able to find materials should not mean a free-for-all, as access to some substances needs to be carefully controlled.
Titian Software has encountered situations where money has been wasted on re-synthesising an expensive material when a colleague had a surplus of it in a nearby freezer – but there was no easy way to know this. In too many cases, finding of materials involves asking around, emailing colleagues, or finding out in project meetings, when a simple search ought to take you straight to the material.
Sample management software should allow you to flexibly search on any number of properties, either across your entire inventory or within specific locations. Ideally, you can save frequently used searches for quick re-use, and to be able to search results for further analysis. It should also be possible to limit the visibility of locations of materials, so the whereabouts of sensitive or valuable samples is only shown to those with the appropriate permissions.
One of the hardest aspects of sample management for a busy laboratory is that of managing expiry or retention dates. There are several reasons why samples may be associated with an expiry or review date, including:
Some of the challenges of managing expiry dates include:
Tracking all of this using only a spreadsheet would be significant undertaking. A good sample management system should give you the tools you need, allowing you to record specific expiry or retention dates against any batch of material, and report on or take appropriate action. For example, you may need to quickly find samples that have reached, or are close to, an expiry date, then list their locations to make it easy to pick and dispose of them.
Alternatively, to re-qualify reagents following Quality Control, a batch of material might be edited to set the new requalification date – and this should automatically be reflected at the sample level for each sample or aliquot of that material. In every case, each of these steps should be fully recorded in an audit trail.
To prevent accidentally using a substance beyond its expiry date, sample management software should clearly display a warning against expired samples at the point of pulling a sample from a freezer.
Laboratory materials are typically stored in a wide variety of containers, for example different brands and sizes of tubes, multiwell plates, flasks, blocks or microscope slides. When managing a sample inventory, it is of course important to track key information such as sample ID, volume and concentration. It is perhaps less obvious as to why it might be valuable to track the type of vessel it is contained in. Here are two real use cases where accurately recording container type information leads to process efficiencies
Fridges and freezers may be physically arranged such that some shelves contain stacks for holding microplates while others may contain boxes holding a particular size of tube. However, researchers may not necessarily know how each store is arranged and how shelves are allocated. The search for relevant space not only wastes time and is frustrating for the scientist but also means freezer doors are being opened and closed unnecessarily.
A good sample management software can address this by allowing you to accurately represent your store layouts to include information on which labware types can physically be stored on which shelves. The software should then be able to guide you to where there is space for the type of container you are trying to store.
So rather than opening many stores to search for appropriate free space for your sample containers, you could let your software automatically assign a space for each of your items. You then just need to put your items away in the suggested locations.
There are several other benefits of labware type tracking, including:
 Lab fridges and freezers become cluttered and untidy over time, making material difficult to find. Even if you know which shelf your sample of interest is on, not knowing the exact tube or rack type can make finding it difficult. This image illustrates how knowing your sample is in a 15mL Falcon Tube would greatly reduce the time and effort taken to locate it.
Lab fridges and freezers become cluttered and untidy over time, making material difficult to find. Even if you know which shelf your sample of interest is on, not knowing the exact tube or rack type can make finding it difficult. This image illustrates how knowing your sample is in a 15mL Falcon Tube would greatly reduce the time and effort taken to locate it.
Reducing the time taken to locate a sample also reduces the time an operator has the door open. This minimises temperature fluctuations that could affect sensitive samples and reduces the build-up of ice within the freezer.
To take advantage of container type tracking, sample management software should allow you to define any type of container to make it easy for you to accurately record this information.
Watch the Video: Sample Not Found
Laboratories are increasingly using multiwell (or microtitre) plates to increase throughput and manage costs through miniaturisation, or take advantage of automation. Screens are also performed in plates, with samples arrayed for a primary screen to check activity, and then hits “cherry-picked” for the next rounds of screening.
Tracking systems which are primarily designed for individual samples may struggle to deal with samples in plates. If you use, or plan to use, multiwell plates, it is essential to choose a sample management system which fundamentally supports them and transfers made between them, whether they be 96-well, 384-well or even 1536-well. This should include tracking and auditing of all changes to the plates and their contents, such as volume decrementing upon transfer, freeze-thaw counting, concentration, volume changes and any other edits.
A tissue microarray (TMA), or multi-tissue block, is an array of many tissue samples embedded in a block, usually of paraffin wax. Each embedded sample is a core removed from a larger sample. The use of TMAs allows higher throughput analysis of many tissue samples at once in a compressed format, while using a smaller amount of material.
Many of the considerations when using multiwell plates also apply to the use of tissue microarrays. Because these may contain human samples, it is essential that the identity of the sample at every position in the array is known. Each sample should also be traceable back to its parent sample.
Where it is important to track the fate of every one of your samples for regulatory reasons, samples in TMAs and multiwell plates must not be overlooked. Using good sample management software you should be able to identify and find such samples just as easily as any other sample in your inventory, thus ensuring that your sample tracking is fully secure and nothing is unaccounted for.
Getting the right information on to your labels can be error-prone when it is done by manually entering data or importing a file into the printer’s software. Sample management software can hold all of this information, and send the correct information for each sample directly to the printer at the time of printing – so your labels are guaranteed to be accurate.
Designing labels should not be a bottleneck in your laboratory. Good software should make it possible to design labels on your screen to match what you will see from the printer.
 You also need to consider the breadth of printer support.
You also need to consider the breadth of printer support.
A good sample management software should be able to print to a very wide range of printers. It should support more than one label size so that you can adapt to the size of the labware you are affixing it to, or include different amounts information depending on the use of the sample.
In Part 2, we explored using expiry dates to help keep your inventory up to date and relevant. Below we look at what happens when a freezer breaks down and how you can still maintain the integrity of your inventory.
A sample management question we are frequently asked is: “how easy is it to reassign samples from one store to another when a store breaks down or is taken out of service?” Some systems just don’t allow for this, or at least make it difficult to do.
When a freezer breaks down, samples need to be physically moved into another freezer quickly, to prevent samples being compromised. Often the most efficient way to do this is to move the contents of entire shelves into an empty back-up freezer. If this is not possible then you may need to distribute racks across existing freezers. Recording these moves manually can be a challenge to do accurately and very time-consuming for operators, which means often it doesn’t get recorded. To make it easy to keep on top of emergency inventory changes, your sample management software should only take a few clicks to move samples from one store to another – either container by container, or at the level of an entire store. All the moves should be recorded in an audit trail so the integrity of your sample record is maintained.
One of the challenges for a facilities manager is being able to notify the owners of materials if a freezer needs to be serviced, replaced, or has broken down. Sometimes the task of identifying the owners of samples in a freezer is done by physically looking through the samples on each shelf. As well as being enormously time-consuming, this puts sample integrity at risk due to the extended time they are out of a store.
Good sample management software should mean notifying owners is not a problem. Rapidly generating a report on who owns the samples held within any store should mean it is simple to notify material owners of freezer changes – planned or otherwise.
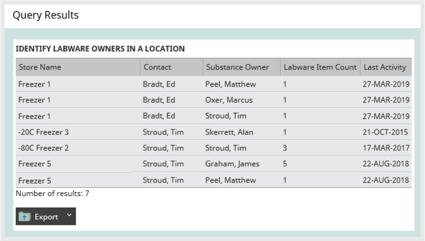
Image: Identifying ownership of samples in your freezers
A common problem for laboratories is that, over time, their inventory has come to be tracked in more than one place. Different groups have different requirements and may tackle the problem in different ways. Samples may be tracked with a combination of paper, Excel and perhaps some home-grown databases. This makes it hard to find a sample, as it requires searching in each individual system. Such problems drive the desire to consolidate on a single system, in order to realise all of the benefits and efficiencies described in previous sections.
Although the case for consolidation may be compelling, frequently the idea of overseeing the transition from legacy systems to a single new system is a daunting one. Areas that may prove complex include data migration, end-user acceptance and good support. Choosing the right system to stay with you into the future is very important, so how do you decide on such a system?
Here are some important considerations when choosing sample management software:
 The adoption of automated storage within life sciences organisations is on the increase, and brings benefits of more efficient use of storage space, accurate retrieval with minimal sample handling, and greater sample security. An organisation’s recognition of the need to improve sample management may sometimes start with the purchase of an automated store.
The adoption of automated storage within life sciences organisations is on the increase, and brings benefits of more efficient use of storage space, accurate retrieval with minimal sample handling, and greater sample security. An organisation’s recognition of the need to improve sample management may sometimes start with the purchase of an automated store.
Although there are many benefits to automated storage, owning an automated store does not solve all of your inventory management problems. It is still necessary to record information about the samples held in the store, which includes substance metadata, volumes and concentrations, and sample history. So, if you have or are considering introducing automated storage for your collection, you will need an inventory management solution that can manage items in both manual and automated stores.
Image: Improving sample management may start with the purchase of an automated store such as TTP Labtech’s arktic
 Integration should mean that your sample management software can create Pick Jobs for the store, and that it is updated automatically when a tube is placed into the store. However, as at least some of your collection is still likely to be in manual storage, the sample management software should be equally capable of managing conventional freezers alongside automated ones.
Integration should mean that your sample management software can create Pick Jobs for the store, and that it is updated automatically when a tube is placed into the store. However, as at least some of your collection is still likely to be in manual storage, the sample management software should be equally capable of managing conventional freezers alongside automated ones.
Image: Good sample management software should seamlessly create orders for automated stores, such as this Hamilton Verso, or for manual storage.
Common paths for expansion include upgrading sample management capabilities to bring in additional features such as:
Sample management software that can separate requests for samples from sample storage and preparation, allows an organisation to set up a cost-efficient centralised sample management operation. Ordering samples should be done via a web interface, as this is both convenient and means new ‘requestors’ do not need additional software.
Image: When upgrading sample management capabilities it is common to incorporate liquid handling platforms such as Tecan’s Freedom EVO
Ideally, any order for a sample in a particular format should be translated into a tracked workflow to manage the fulfilment steps. These steps could be as simple as pick and despatch, but could involve dissolution, dilution, making multiple copies etc. Best of all is if the software can integrate with any automated stores holding the source samples and any liquid handlers being used for dispensing.
This will allow the whole process to be managed from end to end. However, it should still be possible to fulfil any step in the process either by manual or automated operation, in order to give flexibility and to work around instrument unavailability. This also means your organisational can build up its capabilities in stages – beginning with manual processes, later integrating automation for increased accuracy and throughput. To ensure you are not restricted in your instrument choice in the future, you should choose software that has proven integrations with a wide variety of instrument makers.
A sufficiently capable and flexible software solution could be shared by different functions within a single organisation. This further increases its cost-effectiveness and offers you the benefit of fewer platforms to support. It does however require that your software can manage and provide separation between different groups so that they can operate independently.
Choosing inventory management software that can start with the basics and then grow with your operations means never having to start again with new software. This gives you the advantages of:
Too frequently sample management is trusted to spreadsheets or other systems that are inadequate for the task. In this white paper, we point out many common pitfalls and the advantages of avoiding them.
We have set out to show how the adoption of dedicated sample management software can make a positive difference to your organisation through:
All of the examples of sample management “pain points”, and their solutions, are based on real customer examples encountered during the authors’ experience of working in sample management.
1) Good Laboratory Practice Regulations (GLP) 21 CFR Part 58
2) Human Tissue Authority (HTA) Code of practice 9 – Research. https://www.hta.gov.uk/guidanceprofessionals/codes-practice/code-practice-9-research.
3) Denise M O’Hara, Valerie Theobald. (2013) Life cycle management of critical ligand-binding reagents. Bioanalysis 5:21, 2679-2696.
4) Human Tissue Authority (HTA) Code of Practice 9 – Research. https://www.hta.gov.uk/guidance-professionals/codes-practice/code-practice-9-research.
5) Good Laboratory Practice Regulations (GLP) 21 CFR Part 58
Marcus worked for GlaxoSmithKline for 23 years, originally as a scientist working in molecular biology, later transitioning to bioinformatics and then R&D IT.
He joined Titian Software in 2012 where he specialises in addressing the challenges of biological sample management.
Tim worked for Pfizer for 19 years as a scientist in many different groups spanning veterinary medicine, in-vitro pharmacology, reagent provision, structural biology, and compound screening.
He went on to work for MedImmune as a laboratory automation specialist. He joined Titian Software in 2014 as a business application consultant.