This white paper draws on Titian Software’s long running partnerships with multiple vendors of laboratory automation and its extensive experience of implementing sample management systems for organisations of all sizes, plus the author’s 40 years’ experience in the pharmaceutical and software industries.
The principles of good sample management hold for any life sciences laboratory: from a small academic laboratory using manual pipetting methods with human tissue samples, to large multiuser enterprise environments with multiple complex instruments to manage and supply millions of small molecules.
Laboratory instruments are essential tools for reliable and efficient processing, whether that is liquid handling, storage, weighing or HPLC. However, issues can arise with handling each new data stream that these instruments provide.
Integrating your lab equipment with your lab information management system (LIMS) is a powerful step towards digitising your lab, as it provides an error-free data flow which can then be delivered to whichever part of your drug discovery process needs it.
This White Paper discusses approaches to integration and questions to consider. The scenarios discussed relate to labs that are performing sample processing, but in many cases, the considerations are also relevant to other types of lab.
One of the biggest challenges for lab managers is integrating lab instrumentation, tracking samples and auditable inventory updates into efficient physical and digital workflows. This is because:
It is also a huge help to scientific teams – and to your business efficiency – to have a LIMS that will integrate instrumentation and processing with your inventory and audit trail. Efficient laboratory workflows are not just about sample preparation, they also speed up assay cycles and underpin rapid research.
One of the advantages of having a sample management element to a LIMS is that it provides a robust way for scientists to get stored samples ready for use in their assays. However, managing and tracking the process of producing the assay-ready samples is complex. Many physical processing steps need to be carried out (such as neat dispense, solubilisation, liquid transfer), each of which may involve different equipment and quantities.
The sequences of processing steps are often referred to as workflows, and they take multiple factors into account to ensure that what the scientist has requested can be satisfied. For instance:
Ideally, a LIMS includes workflow management, which can validate the workflow and track progress while it is carried out. If a workflow fails validation, then the LIMS should feedback that it is not possible and why.
The range of equipment in your labs and how you want to use it will be unique to your company’s setup. For this reason, there is no one answer to how best to integrate your laboratory equipment with your LIMS. Indeed, how quickly you wish to deploy a solution and/or your budget may have a significant impact on which options you consider first.
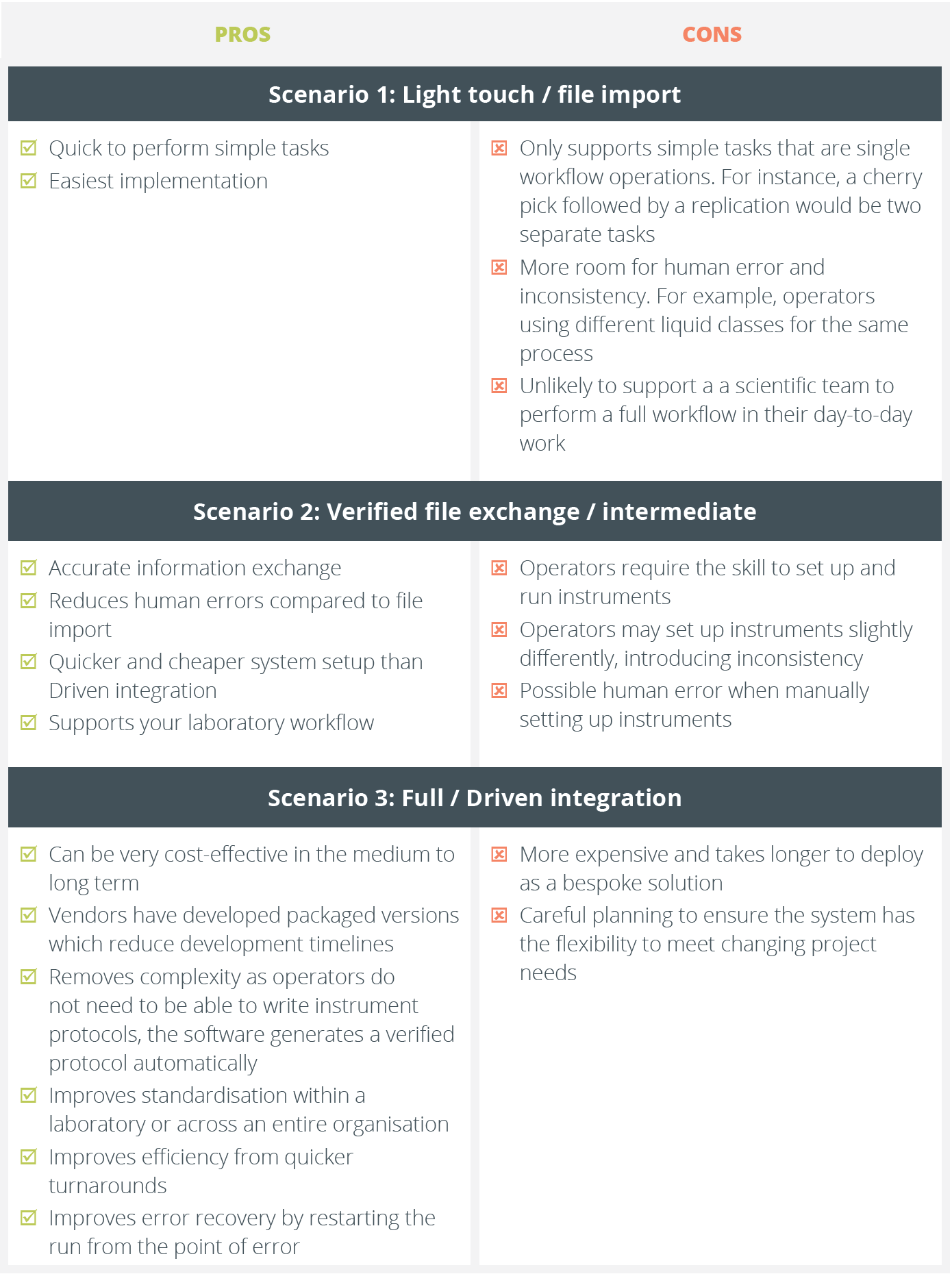
Equally important to realise is that there are different levels of integration, to suit different needs and budgets. We have classified them as follows:
Scenario 2: Workflow-led integration by verified file exchange. When your LIMS has a workflow, this allows for a two-way communication integration where operators get guidance from the LIMS of what is required in clear workflow steps. They write the protocols for instrumentation and a verified file exchange process, which is frequently automated, creates pick list files for the protocols and imports verified data into your LIMS to update the inventory.
Scenario 3: Driven instrument integration. A tightly integrated or driven integration is where your LIMS ‘drives’ instrumentation directly through the equipment’s Application Programming Interface (API) and creates the protocols required. The two-way data transfers are automatically managed and verified to update inventory and audit trail in real-time. This level of integration is highly efficient but complex, effectively bringing equipment from different automation vendors into one seamlessly managed system. For this reason, it costs more and takes longer to deploy, but it brings swift returns on investment for busy labs through error reduction as well as efficiency.

The simplest integration of all is to do everything in a manual manner. This can be very powerful for some situations that do not require a workflow, and may actually not include any laboratory equipment. Examples of this include:
The simplest file transfer integrations are one way, only tracking individual steps and not more complex tasks, such as a cherry pick and serialise. They do not give you an overview of the whole workflow or provide guidance to the instrument or operators.
The overall aim of integration is to make both your laboratory equipment and your laboratory processes more effective. This white paper explores the pros and cons of these different levels of integration for the following equipment:
The aim of these examples is to help you consider the degree of integration needed when examining your workflows, whatever your scale of operation.
The integrations discussed in this white paper are not mutually exclusive. Your requirements may include elements of all of them. Indeed, your current needs may suggest you start at the minimum viable level for you and upgrade to a closer, more driven integration in the future.
What is important is that you take time to ask the questions pertinent to you and your organisation. Because there are different levels of integration, you should ensure that what you are considering matches your expectations and requirements for functionality, as well as how it will fit into your lab. Time taken now will save you money, speed up deployment and help a smooth transition. This white paper aims to help you to formulate your questions.
There are a wide variety of liquid handlers used in labs and they can perform complex tasks. Some are completely custom, but most are standardised and so are likely to have APIs (Application Programming Interfaces) that provide an easier route for tighter integration. Some level of integration is always possible, which we will explore using our three scenarios:
As mentioned above, simple file import integration is best suited to non-workflow driven processing for simple repetitive tasks, such as solubilisation of samples to a standard volume and placing them in a store.
For liquid handlers, this might be when you are comfortable writing your own protocols/ scripts to run the equipment for your own processes, but you still want to track the samples created, plus update the source samples. For example, if you are running simple replicates where you can write your own protocol to run the liquid handler. It is good practice to update the source data when adding the new samples and their history to your inventory.
Inventory information can be updated and recorded in your LIMS by importing a data file which contains the transfer information from source to destination. While it is simple to create a process to import data files, do not forget the file needs to be verified for errors (such as trying to transfer more volume than is available in the source container or using a barcode that is already being used in your inventory). Error checking can be the difference between an integration that works and one that needs continual editing of the data.
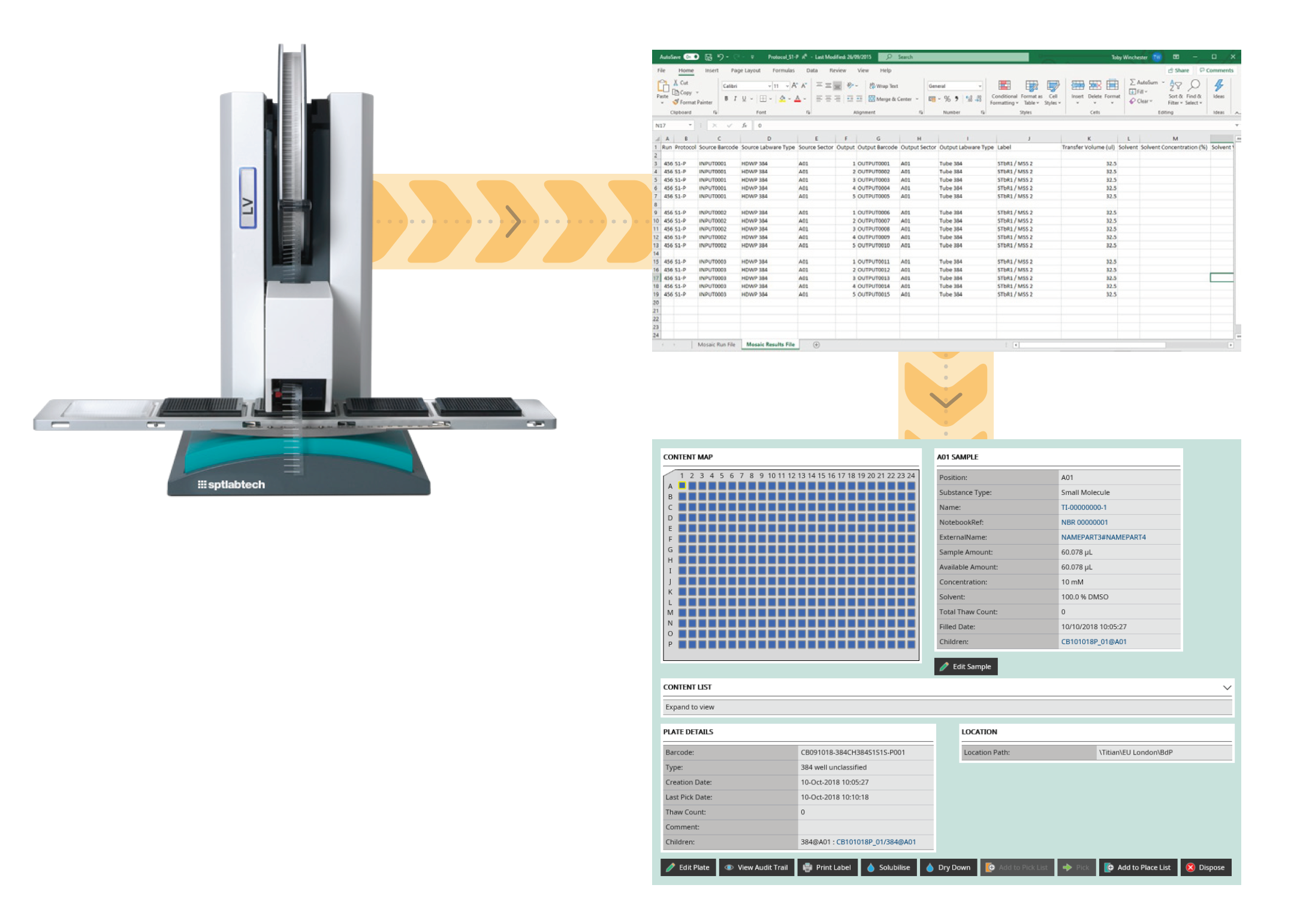
The imported data file should also allow you to record the instrument used to do the work, how much substance was consumed, any transfer failures, sample parentage, when it was done and by whom. This information is essential to create an audit trail, which may also need to be 21 CFR part 11 compliant. Having a full audit trail information in your LIMS helps to troubleshoot, should a problem be spotted.
In addition to a verified file import of transfers, your sample management LIMS should allow you to create new samples directly from a source within the user interface. For example, to create child tubes and plates, or to solubilise/dry down tubes and plates. Again, this process should include full audit trail information.
Questions to consider when considering file import integration for liquid handling processes are:
Consider performing a more complex task where you want to solubilise a sample to make a tube stock solution (and store it), create a plate from the solution stock, replicate the plate and dispatch the replicated plate as well as tracking all the changes as they happen.
While each these actions could be tracked using the file import integration from scenario one:
This is where the workflow management component of a LIMS becomes of real value.
Workflows and LIMS
With a workflow in place, it is possible to validate in advance if the sample processing is physically achievable, for example:
As well as providing validation, a workflow can be used to guide the operator through the process steps required to complete the work. As each step is completed, the workflow will update inventory and adjust subsequent process steps as appropriate.
For example:
File exchange and LIMS
Verified file exchange integration allows accurate two-way communication between your LIMS and laboratory instrumentation, whilst the operator still sets up and runs the instruments.
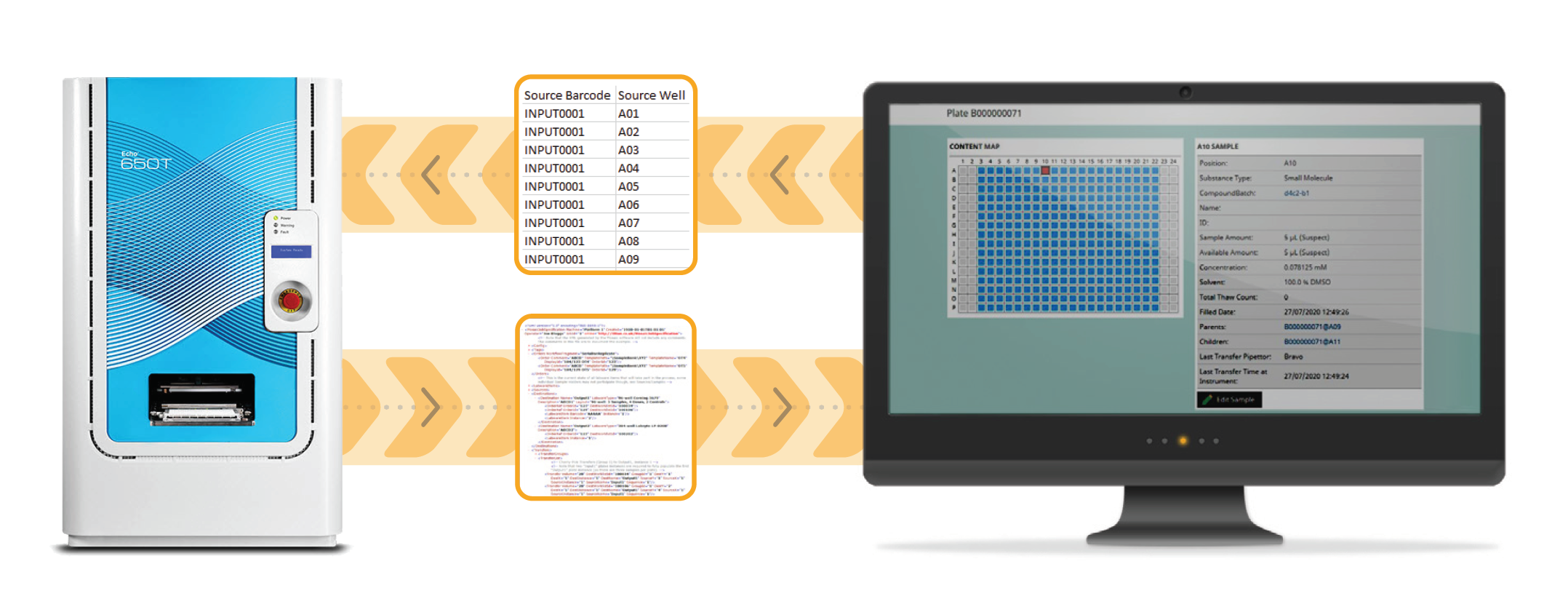
The steps involved are:
Because of the large variety of vendors and liquid handling instruments available, there is a move to using standardised generic Export/Import file formats for file exchange integration. However, if this file format does not fully fit your needs, there are multiple file manipulation software options available to create custom file manipulations to customise things to your exact requirements.
File exchange plus
Whilst verified file exchange will commonly use generic formats, if a specific instrument is very common you could expect to import that instrument’s output file directly into your LIMS. This integration could be considered as file exchange plus. A good example would be the Beckman Coulter Life Sciences Echo platform, which is widely used across the life sciences industry. The Echo produces a standard XML output file across its complete range of applications. A verified file exchange plus integration would import these files directly and update inventory accordingly, removing human error in updating inventory.
Questions to consider for verified file exchange integration:
Liquid handlers can perform multiple operations in a single run, such as solvent addition, mixing and solution transfers, as well as possibly containing other integrated devices. Such systems require complex setup in order to perform a run. In a busy lab, often what is needed is the ability to walk up to an instrument, be prompted for the required labware, push a button, and then walk away knowing that your LIMS has worked out all the transfers and volumes for you, and instructed the equipment on how to run following your standardised methodology.
Driven integrations replace a lot of manual work by automatically calculating what should be done by each liquid handler and directly providing exact pipetting instructions, including volumes, sources and destinations for transfers, as well as monitoring success or failure and handling overlapping workflows.
In driven instrument integrations, the LIMS software communicates directly with liquid handler to provide instrument protocols based on the workflow. Data from the instrument is picked up in real time. However, the instrumentation must have an API to allow two way communication, such as those provided by Tecan, Hamilton, SPT Labtech, HighRes Biosolutions and so on. The parallels between file exchange and a driven integration will also apply to a wide range of laboratory instruments with an API.
Examples of file exchange and driven integrations include the Beckman Coulter Life Sciences Echo acoustic liquid handlers. The standalone Echo can only be integrated to the file exchange plus level as it lacks an API. The Access robot system acoustic liquid handler platform adds an API (via the Tempo software) making tighter direct integration possible.
A fully driven liquid handler workstation integration should be able to:
Work across a variety of similar instruments from different manufacturers
Standardise methodology across multiple instruments and/or sites to minimise process variations
Determine if two or more orders have compatible requirements so they can be processed together, to improve efficiency
Handle failures (due to a power cut, for instance) so that a run can quickly be resumed and that your inventory is only updated with the transfers that actually occurred
All of these will result in in overall efficiency benefits.
Producing a driven integration, with its real-time two way communication between systems, takes time to analyse, plan, collaborate, develop and deliver and is thus expensive as a bespoke option. However, many of these integrations have already been done by vendors and are available as packaged solutions which optimise development timelines.
The tight coupling between instruments and LIMS required for driven integration is sometimes viewed as inhibiting the process flexibility required to meet changing project needs. However, with good planning, this should not prove to be an issue. Making sure your driven system can cope with the flexibility that you are actually going to encounter is something that should be reviewed during the integration selection.
Questions to consider for driven integration are:
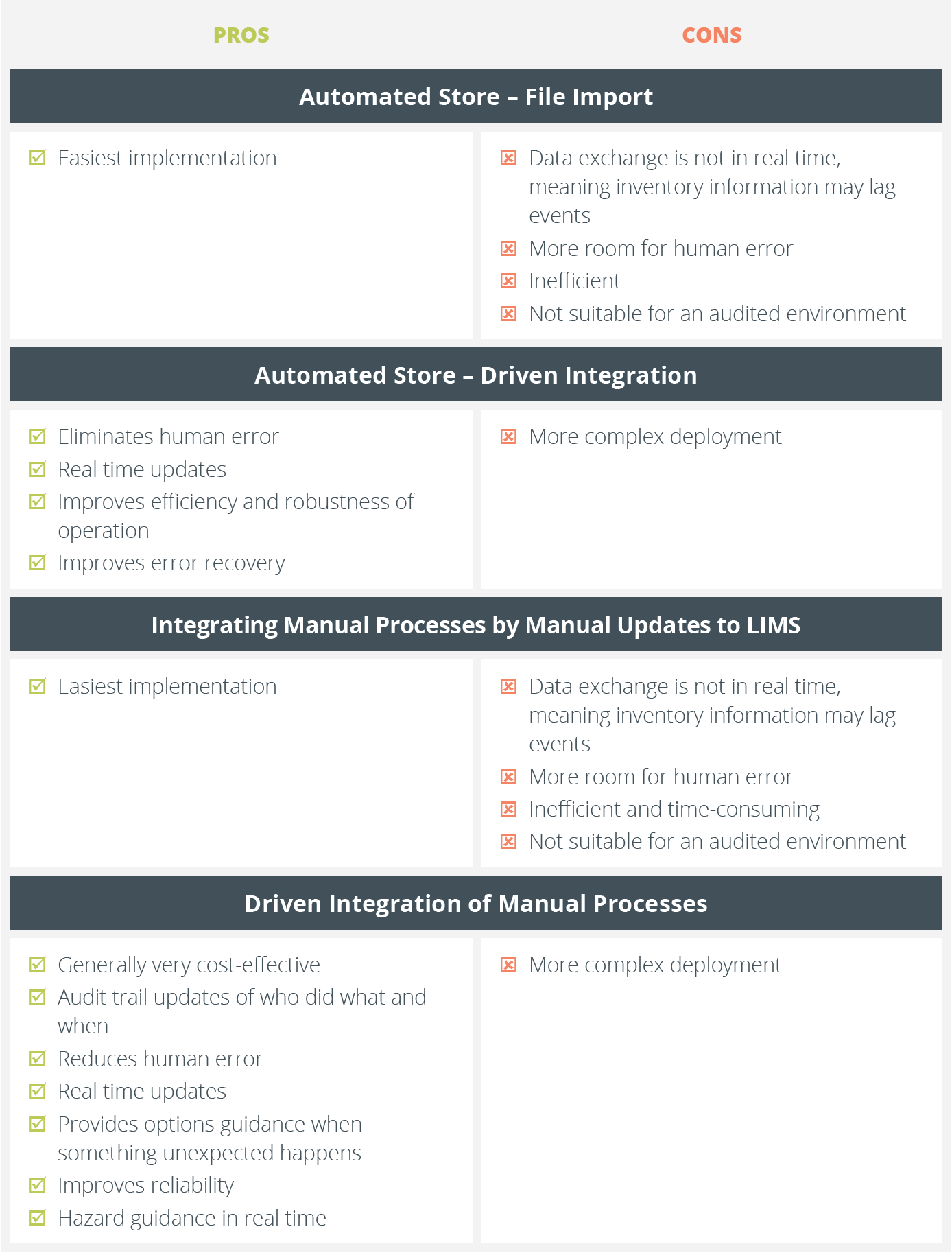
Automated stores provide automated sample storage with high throughput sample access, greater space efficiency and improved sample integrity when compared to manual freezers. Automated stores have simpler inventory management requirements than liquid handlers and so the integration options are simplified to:
In this simplest case of integration, the placing of labware into a store could be tracked manually by importing a file containing a list of barcodes for all the items added to the store plus their new locations. The process would entail:
To physically pick samples from a store, all the store needs is a file containing a list of barcodes to pick. A LIMS system can easily provide this from a sample request.
However, the data file needed to update your LIMS inventory after picking is more complex. In addition to the tube barcodes, your inventory needs to know the rack barcode and the location of each tube within it.
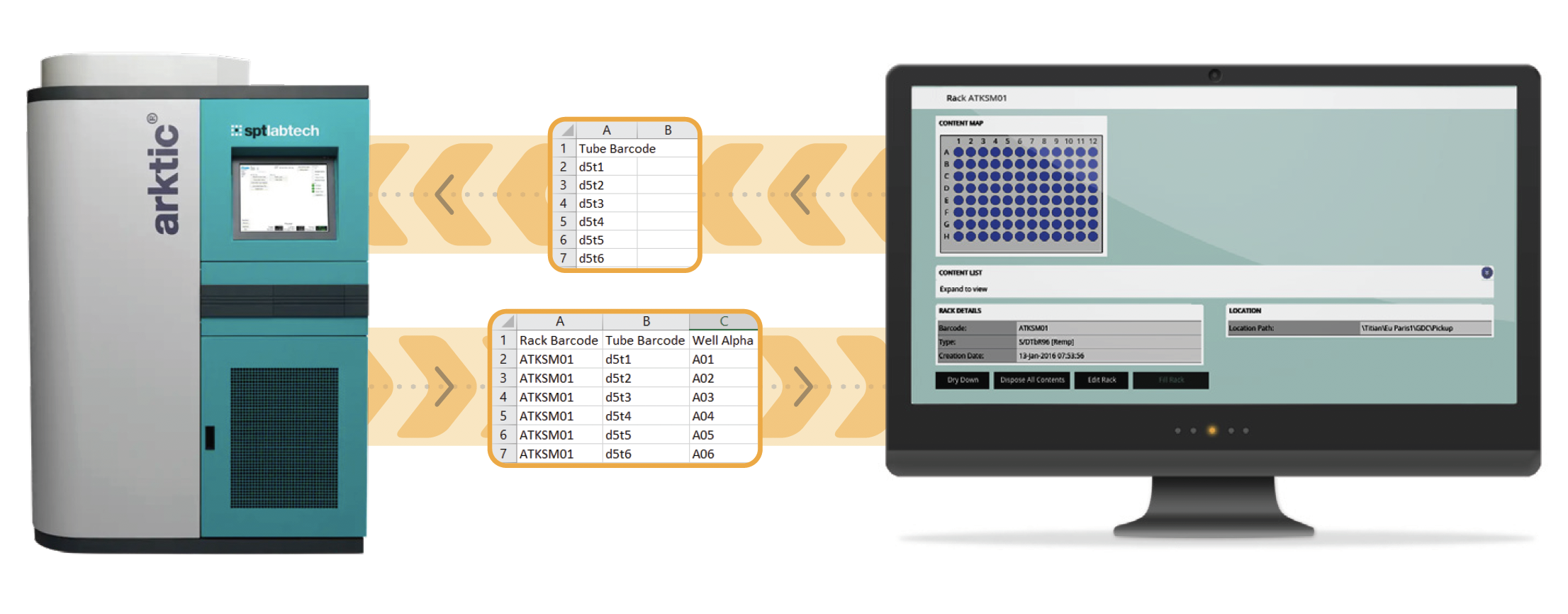
Using manually created files to update your inventory is problematic for two reasons:
Inventory updates that are not done in real time have potentially serious consequences:
It is possible to run an automated store, particularly a low throughput one, using the manual file import method but it is not often logistically effective to do so. Having a driven integration smooths your workflow and brings with it several advantages:
A fully driven automated store integration should also improve the robustness of your operation. A good integration is designed to handle a wide range of errors and aid their rapid diagnosis and resolution with a minimal amount of user input. Most common problems arise from human error.
For example, when loading racks of tubes into store, a LIMS can automatically cross check that tubes are present in the racks and inform operators if something unexpected is found, such as unregistered labware. For ‘non-blocking’ errors like this example, the LIMS can instruct the store to park the rack causing the error and continue placing other racks. The operator can then connect to the store (usually remotely) to resolve the issue and finish the process.

Questions to consider when considering manual file import versus driven integration for an automated store are:
The push to digitalise and track all the pieces of the laboratory process means that manual processes need to be included in integration plans for labs. It is already quite common for some tasks: for instance, manual pipetting can be done following guidance from your LIMS user interface, which gives position information and automatically calculates volumes to minimise human error.
Other processes requiring the operator to manually perform a task can also directly involve the use of laboratory equipment, such as weighing with analytical balances, checking tube positions with rack scanners, or placing and retrieving samples from manual stores.
These manual processes are usually simple compared to the multi-step workflows carried out on automated liquid handling platforms. However, achieving an integration that guides the operator in such a way that it removes human error still requires a degree of effort that should not be underestimated.
Integrating Analytical Balances
Weighing is predominantly done as a manual process with an operator sitting in front of an analytical balance. The process is usually viewed as extremely simple, with the operator just needing to know how much to dispense. So why does this process need integration? There are several strong reasons:

A simple integration using verified file import can be used to update your inventory with balance information, tare and gross weights while avoiding unnecessary contamination of surfaces. However, it does not address process consistency.
A driven integration between your LIMS and analytical balance will directly transfer accurate readings to your inventory in real time but also provide:
Handling for unexpected events. For example, tracked options on how the operator should proceed if the sample to be weighed is a gum as opposed to a free-flowing powder, or the source bottle is damaged[1]
Recording and enforcement of SOP processes, such as taring labware or check weighing after a defined number of dispenses
A 21 CFR Part 11 compliant audit trail of dispenses for every vial
Label printing to deliver human readable information to the recipient
Questions to ask yourself when considering a file import integration versus a driven one include:
Integrating Rack Scanners
Rack scanners provide a quick way of tracking tubes by scanning racks of 2D barcoded tubes and providing a list of their positions and barcodes to your LIMS, usually as a CSV file. Scanners are mainly used to introduce new inventory quickly, track manual movements of tubes or to recover after a rack is dropped.
A validated file import integration means that the scanned tube locations can be updated in your inventory, but this is all.
A driven integration between a rack scanner and your LIMS allows the LIMS to provide guidance and alerts when action is needed. For instance, it can highlight:
The guidance for operators that comes with a driven integration can be invaluable for reducing human error. For instance, if a rack has been scanned 180° wrong, in a file import integration, the inventory would be updated with incorrect location information. If this rack was then sent to a liquid handler, the robot will try to pick from H12 to get the sample in A1. A driven rack scanner integration would flag the inconsistency to the operator.
Integrating Manual Stores
A critical task for sample management LIMS software is to create an accurate digital twin representing the hierarchy of your manual laboratory storage units: cupboards, fridges and freezers. This allows users to quickly search and locate stock as well as finding free space for placing new samples. Maintaining the accuracy of this inventory and providing an audit trail of who did what requires some level of integration.
The manual process involves the operator using the LIMS user interface to create a list of what samples are needed (maybe on a piece of paper), then collecting or returning them, and finally updating their locations in the inventory. These updates do not happen in real time and it is a common problem to have items returned to the wrong place or the user forget to register the new location.

New technologies enable a driven integration between a LIMS and manual stores by enhancing the LIMS user interface using either:
Both devices bring together real time labware barcode verification and context-specific operator guidance from the LIMS. This offers the following benefits:
These new technologies also offer efficient processing as operators are immediately directed to the next task, without needing to return to a computer.
Questions to consider when reviewing integration options for manual stores include:
Summary of Integration Types
Other Factors to Consider
So far this white paper has mainly discussed technical and functional aspects of digitising sample management processes to integrate with your LIMS, but there are other considerations too.
Ask about support and consider how the integration may evolve as your company grows or processes change. If an integration is entirely custom to you, then it will require unique support, which is more expensive in the long run. If it is an existing product, or based on one, then it is often supported globally at reduced cost and is likely to be kept up to date. There should be an upgrade route to allow for new versions of software, or to upgrade instruments. Will you have a team to support your integration in the future, or is it dependent on one person who may leave the company? Is support local or worldwide, and which matters most to you?
Consider deployment. While a driven integrated system requires more setup than a verified file exchange system, some work may be required by you even for verified file exchange integrations. The key is being able to pass files of information smoothly between your systems. In a few cases, data can be used directly by both the instrument and LIMS. When this is not the case, many LIMS offer a generic export/import file format which can be used as the basis for integration. If the LIMS file format does not fully fit your needs, there are options to adjust it without requiring a completely bespoke approach. Various file manipulation software options are available, including vendors who will create custom file manipulations to meet your exact requirements. If bespoke development is your route, then ask to work in a SCRUM way with regular reviews of progress, so you are aware of how easy the system is to work with.
It is important to consider change management when implementing levels of automation and integration. Staff may be concerned that their roles will no longer be interesting or valued and thus be resistant to new processes.
Integrations of manual processes can be a useful first step to further automation integration, because they can help staff to accept the value of changes. Often, these manual processes are tedious as well as error prone. Integrations that make these tasks easier for staff, as well as speeding them up and removing errors, will improve user satisfaction.
All the types of integration discussed in this white paper have their uses in capturing data, but each provides a different level of functionality. When discussing your integration needs – with vendors or inside your company – it is important to be clear about what level of integration you are offered. You should have in mind what functionality is important to you and make sure that you fully understand whether this is covered.
When considering integration options for each process, think carefully about how varied the process is rather than the number of samples handled:
Integrating manual processes with your LIMS brings significant benefits because it brings reliability and traceability to processes that can be prone to errors. For instance, driven instrument integration with analytical balances or rack scanners is likely to be a cost-effective way of improving data quality, irrespective of your overall level of integration.
Considering the human element of integrations is also important to ensure that staff are happy and productive. Digitised processes need to be streamlined and easy to use so that operators recognise the gains from integration. Ask for demonstrations to both operators and lab managers to see how some examples of your processes could be handled. If development is required ask for regular SCRUM demonstrations of progress.
Providing a robust integration to suit your needs will require a partnership between your laboratory, your sample management team, the automation vendor and the integration experts – whether internal or external. The requirements of both lab and sample management teams need to be addressed, as well as the capabilities of the automation. All parties need to be committed to working together, to ensure that information flows smoothly and you can meet the timescale and budget you expect.
DOWNLOAD THE PDF VERSION OF THIS GUIDE
Roger worked for GlaxoSmithKline for 33 years.
Originally a medicinal chemist working as a research chemist, he later transitioned to cheminformatics and then R&D IT, specialising in compound management software.
He joined Titian Software in 2012 where he is a technical application consultant.